Cellex, Inc qSARS-CoV-2 IgG/IgM Rapid Test Cassette
The Cellex, Inc. qSARS-CoV-2 IgG/IgM Rapid Test, which has received an Emergency Use Authorization (EUA) from the U.S Food and Drug Administration (FDA), is indicated for the qualitative detection and differentiation of IgM and IgG antibodies against SARS-CoV-2 in serum, plasma (EDTA or citrate), or venipuncture whole blood from individuals suspected of COVID-19 by a healthcare provider. Emergency use of this test is limited to authorized laboratories.
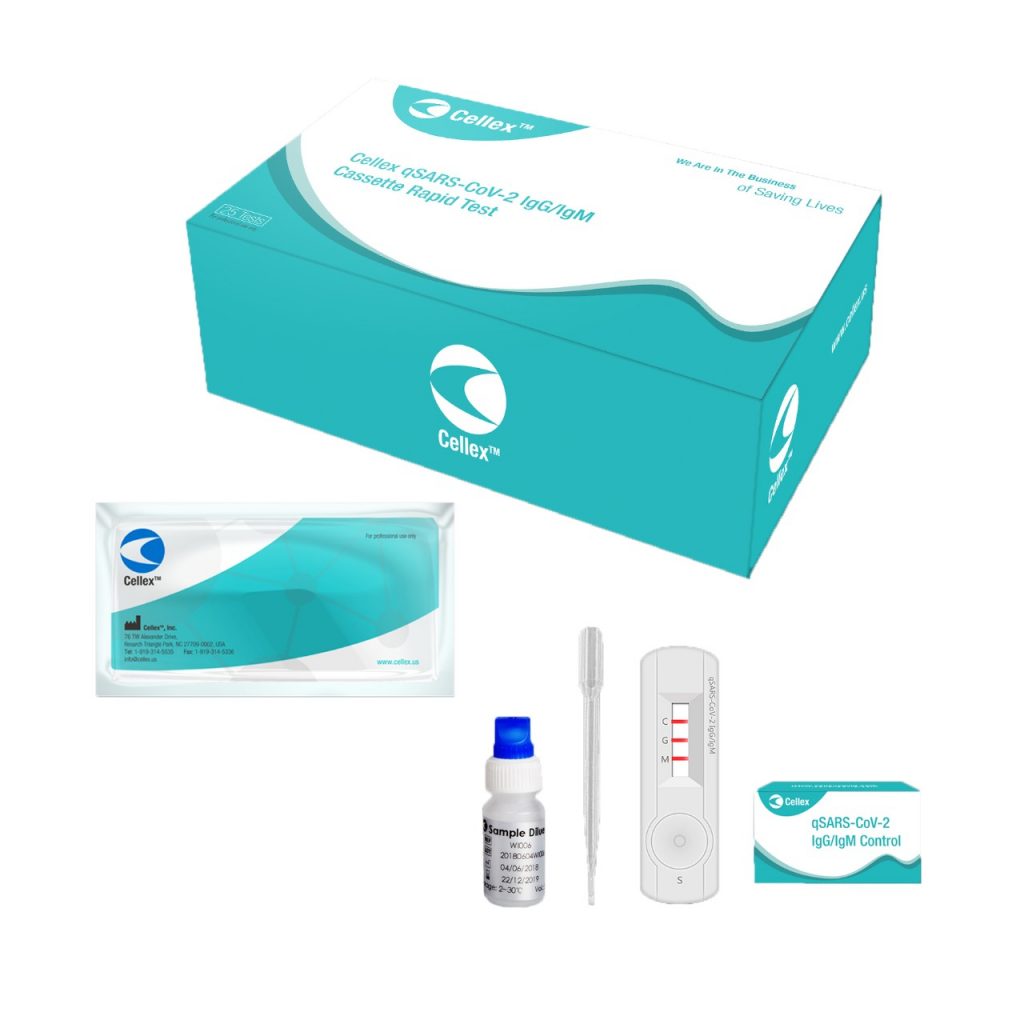
Test Type: Rapid Antibody (IgM/IgG)
Specimen Type: Serum, plasma (EDTA or citrate), or venipuncture whole blood
Turnaround Time: 15-20 minutes, results are not valid after 20 minutes
FDA Status: EUA granted
CLIA Complexity: Moderately/highly complex
Ordering information:
Contact your Compassionate Assistance Products Representative to order or e-mail us at [email protected]
| Material # | Description | Pkg string |
|---|---|---|
| 5515C025 | qSARS-CoV-2 IgG/IgM Rapid Test Cassette* | 25 kits/carton, 400 cartons/unit |

Recent Comments